As with medical systems that include software systems, manufacturers face the same challenges: time, quality, scale (quantity and complexity of functionality), and cost. In addition, products are subject to approval by local regulatory authorities such as the US Food and Drug Administration (FDA), the European Medical Device Directive (MDD), the British Food and Drug Administration (MHRA), and other similar regulatory agencies.
In this article we will explore how dynamic code analysis can help medical devices demonstrate security compliance and the key capabilities of dynamic analysis tools. To help designers choose an operating system (OS), the article also briefly describes what features of the OS can drive security-related software to accelerate design, development, and approval processes.
Expertise and process
Expertise and a good development process do not ensure that the system meets the reliability required, or even that it is a good system. But the two can indeed greatly increase this possibility.
The succinct design required to create a safety-critical system requires superior expertise. To demonstrate that the software system being tested meets the security requirements, a thorough understanding of the software verification method, the software being evaluated, and the evaluation environment (including verification of similar systems) is required.
There is no doubt that the IEC 62304 standard focuses on the development process. With this in mind, our work will be better, not only in the context of meeting the most stringent quality management standards, but also in using tools to help ensure that our systems meet these standards and to auditors. And the regulatory body provides evidence to prove it.
Demonstrate reliability
To ensure regulatory approval, manufacturers must demonstrate that these devices meet safety specifications. For device software, verify that they meet the requirements of the Trustworthy (Reliability and Availability) standard. Whether it is to meet reliability or availability requirements depends on the usage of the system. Detailed requirements limits and precise credibility requirements provide established prerequisites and precise methods to help us verify the credibility of our software systems.
Define acceptable risk
No software system is absolutely reliable. Even if the system is absolutely reliable, we can't prove it. The available methods do not prove that the system will never fail, they can only help us find and avoid the occurrence of errors, and estimate the possibility of failure. Therefore, when the failure rate of a software system is low enough and there is no unacceptable risk, it is "safe." The precise meaning of “unacceptable risk†or “acceptable risk†varies by industry and administrative jurisdiction. Measurement methods include:
Ø ALARP (As Low as Reasonably PracTIcal): Define and classify potential hazards and associated risks as: a) clearly unacceptable, b) if the cost of removal is too high, then tolerable, and c) accept. All unacceptable risks must be removed, but the risk can be tolerated only if the removal cost and time are reasonable.
Ø GAMAB (globalement au moins aussi bon) or GAME (globalement au moins équivalent): The risk level of the new system should be at least roughly equal to the risk level of the existing system.
Ø MEM (Minimum Endogenous Mortality): In the field of new system deployment, it can not exceed one-tenth of the mortality rate in the region. For example, in Western countries whose age is 20, this value is about 0.0002.
All of these methods need to be adjusted according to the actual situation, mainly depending on the number of people whose serious faults may be affected by the equipment. When using the ALARP approach, in order to determine which risks are unacceptable, which are tolerable, and acceptable, we need to determine the maximum probability of failure allowed for a severe failure for each risk. And if you use the GAMAB and MEM guidelines, we need to determine this value globally.
Method to prove software reliability
At present, there is no single method sufficient to prove that the software system meets the reliability requirements. Therefore, our reliability demonstrations must incorporate a variety of methods and techniques, including but not limited to:
Ø Development environment in accordance with IEC 62304 and other similar standards
Ø Require a tracking matrix to ensure that all safety related requirements have been met
Ø Formal design methods and tools can provide a mathematical basis for the correctness of the design
Ø Fault tree analysis using Bayesian confidence network method
Ø Retrospective design verification, evaluation of system design based on completed work
Ø Static analysis, using model detection or data flow analysis
Ø Test using direct fault detection technology, such as dynamic analysis, identify faults by errors and failures
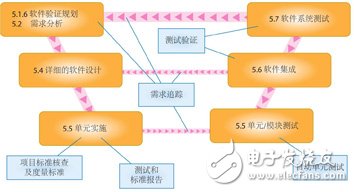
Figure 1. The different analytical methods and related chapters covered by the IEC 62304 standard are presented as a typical "V" shaped development model. Each method shown in the figure does not depend on the process. Any other development process model can be similarly expressed: waterfall, iterative, sensitive, etc.
Our company is specialized in supplying wire tube condenser,Fridge Condenser,refrigeratory condenser.Material: Bundy tube (steel tube coated with copper): 4x0.71mm,4.76 x 0.71mm ,6x0.71mm,6.35x0.71mm,8x0.71mm. Steel wire: 1-1.6mm . Copper filter driers . Structure: bundy tube with steel wire . Painting: electrophoretic painting (black) .We can supply the items according to customers' drawings or samples .Besides. we use the hydrolysis technique to ensure whether the condenser will not leak. Fridge condenser is very popular in the after sales market for freezers , coolers and refrigerators .
Fridge Condenser
Fridge Condenser,Condenser Refrigeration,Freezer Condenser,Refrigerator Condenser
ZHEJIANG ICE LOONG ENVIRONMENTAL SCI-TECH CO.,LTD. , https://www.china-refrigerantgas.com